Select All the Statements That Correctly Describe Compounds
Some soluble exceptions include Naco K_CO and NHCO All ionic compounds containing the sulfide anion are soluble in water. All ionic compounds containing the nitrate anion are soluble in.

Che 140 Ch 6 Learn Smart Flashcards Quizlet
The most stable chair conformation for the trans isomer has both CH3 groups in equatorial positions.
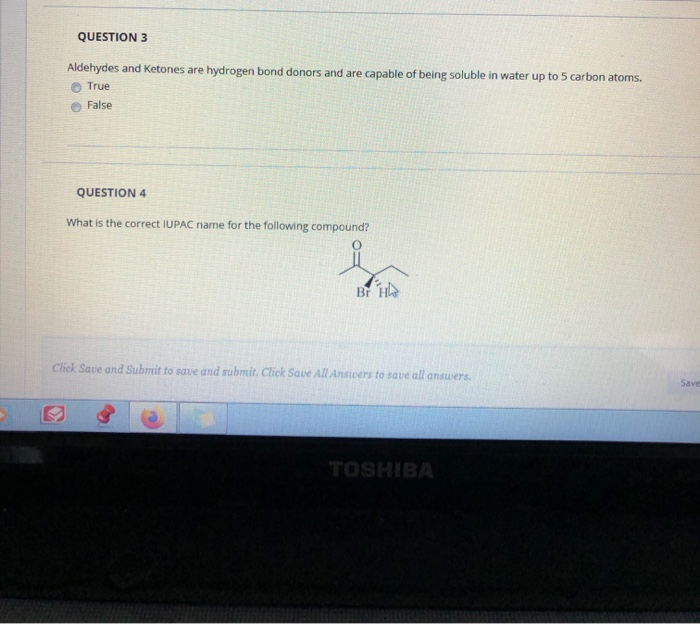
. Aliphatic HCs are shorter than aromatic compounds. Are components of culture. Select all statements that correctly describe diastereomers.
Select all statements that correctly describe the acid-base chemistry of aspirin and its biological implications. Matter forms when particles chemically bond together. Aromatic HCs are double bonded while aliphatic are single bonded.
According to solubility rules two soluble ionic compounds could not both dissolve in the same sample of water. Slide Check all that apply. Diastereomers have the same molecular formula.
Designate the configuration of the stereogenic center in the structure shown as R or S. They have a polar covalent bond which makes carbon a nucleophile and an electrophile D. What statements correctly describe organometallic compounds.
Matter may exist as a solid liquid or gas. Aromatic HCs contain a benzene ring while aliphatic do not. Select all statements that correctly describe the reduction of this compound.
HF Select all statements that correctly describe the compound formed between P and Br atoms in which the P contains one nonbonding pair of electrons. - orbitals containing electron pairs in covalent bonds at a central atom are oriented to minimize electron-electron repulsions. Matter is made up of atoms.
Select the element or compound in which the electrons of a covalent bond are unequally shared. Li2O Select all the polar molecules. The rules used to predict the.
Aliphatic HCs have H and C atoms only while aromatic have other atoms like O and N. Most carbonates are insoluble in water. The highest priority group is Br followed by Cl and then the methyl group.
Select the lithium compound whose formula is shown correctly. Select all that apply Which of the following statements correctly describe compounds. Which statement is TRUE about aliphatic hydrocarbons and aromatic hydrocarbons.
Select all that apply. Including holidays and customs. Covalent compounds are formed by transferring electrons from one atom to another atom Most of the compounds that we come in contact with are covalent compounds Covalent compounds are formed by sharing electrons between atoms.
Select all that apply. A Lewis acid is an electron pair acceptor while a Lewis base is an electron pair donor Select all statements that correctly describe Lewis and Bronsted-Lowry acids and bases. Diastereomers are not mirror images of each other.
Regardless of quantity an ionic compound that is soluble according to solubility rules will dissolve in water 100. Check all that apply. Nitrogen Ny is a covalent compound.
Binary ionic compound typically forms when a metal reacts with a nonmetal. They are transformed into hydrocarbons by reacting with CH3OH or CH3COOH C. Binary ionic compound forms by the sharing of.
Select all the statements that correctly describe solubility rules for ionic compounds in water. Select all the statements that correctly represent the basics of VSEPR theory. Select all that apply.
- lewis structures combined with VSEPR theory allow us to predict molecular shapes and bond angles of molecules and polyatomic ions. Consider the stereoisomers cis-12-dimethylcyclohexane and trans-12-dimethylcyclohexane. Check all that apply.
Matter is a measure of the amount of material in an object. 2 Get Other questions on the subject. Which of the following statements correctly describe the formation of binary ionic compounds.
Select all the statements that correctly describe the solubility of ionic compounds in water. Which of the following statements correctly describe compounds. Covalent compounds contain covalent bonds.
O 1 equivalent of H2Pd-C would reduce only the CC groups o NaBH4 would reduce the CO group o Excess H2Pd-C would reduce both the CO and CC groups LiAlH4 contains a more polar metal-hydrogen bond than NaBH4 and therefore is more reactive in reduction reactions. Select all the statements the correctly describe these two compounds. The cis isomer has two chair conformations of equal energy.
Check all that apply. They have an ionic bond because the bond is between a metal and a nonmetal B. Questions in other subjects.

Solved Question 1 In A Carbonyl Group A The Carbon Atom Chegg Com

Che 140 Ch 8 Learn Smart Flashcards Quizlet

Che 140 Ch 7 Learn Smart Flashcards Quizlet

Che 140 Ch 6 Learn Smart Flashcards Quizlet

Solved Question 16 Choose All That Are True For Sn1 Chegg Com
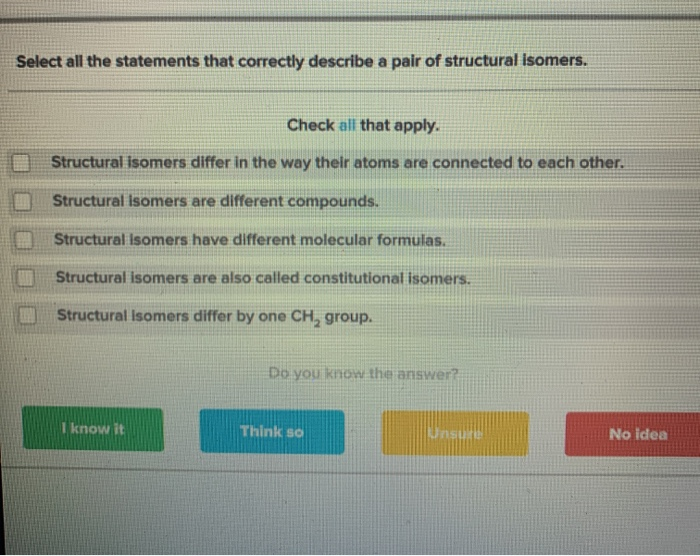
Solved Select All The Statements That Correctly Describe A Chegg Com

Solved What Of The Following Options Describes The Term Chegg Com

Che 140 Ch 7 Learn Smart Flashcards Quizlet

Che 140 Ch 7 Learn Smart Flashcards Quizlet

Question Video Selecting The Statement That Does Not Describe Ionic Bonding Nagwa

Che 140 Ch 8 Learn Smart Flashcards Quizlet

Che 140 Ch 8 Learn Smart Flashcards Quizlet

Che 140 Ch 7 Learn Smart Flashcards Quizlet

Che 140 Ch 8 Learn Smart Flashcards Quizlet

Solved Question 23 Match The Nonmetal With The Anion It Chegg Com
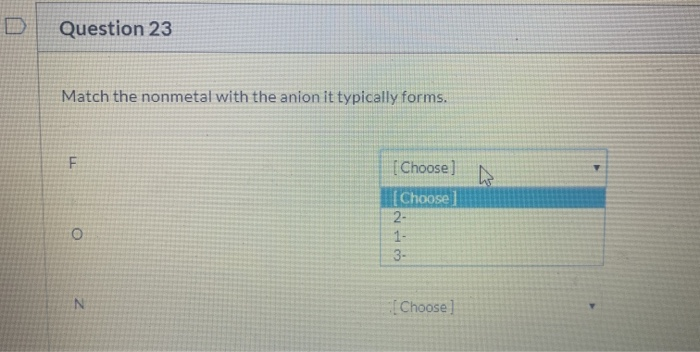
Solved Question 23 Match The Nonmetal With The Anion It Chegg Com

Che 140 Ch 6 Learn Smart Flashcards Quizlet

Solved What Of The Following Options Describes The Term Chegg Com

Comments
Post a Comment